Gazette 3, August 2019

Welcome to the August 2019 Glaukos Gazette
WORD FROM THE GM
Dear Surgeons,
We often get asked about new data.
Adding to the robust clinical effectiveness and safety evidence available on iStent Technologies are two new recent publications on iStent®.
- A prospective, randomised trial highlights 5-year performance of 2 standalone iStentsvs. topical prostaglandin as an initial intervention in newly diagnosed glaucoma patients (Fechtner RD et al, Ophthalmology Glaucoma 2019). A total of 101 subjects were randomised in a 1:1 ratio to receive either two iStents in a standalone procedure or once-daily topical travoprost.The iStent cohort achieved significantly higher treatment success and significantly fewer iStent subjects required add-on medications at 5 years postoperative, compared to topical prostaglandin subjects.At five years, results showed:
- Mean diurnal IOP was 16.5 mmHg (35.3% reduction; p < 0.0001) for the iStent group vs. 16.3 mmHg (35.1% reduction) for the travoprost group, excluding eyes in both cohorts that underwent cataract surgery during follow-up.
- Treatment success – defined as mean diurnal IOP of 6 mmHg to 18 mmHg without add-on medication or secondary glaucoma surgery – was achieved in 77% of stent eyes vs. 53% of travoprost eyes (p = 0.04).
- 17% of iStent eyes vs. 44% of travoprost eyes required add-on medication.
- The need for add-on medication arose at a slower rate in the iStent group than in the travoprost group, especially after two years of follow-up. Study authors observed that from two to five years of follow-up, add-on medications were initiated in roughly double the number of travoprost eyes vs iStent eyes.
- The safety profile was excellent in both groups throughout follow-up.
- A prospective, non-randomised, consecutive case series confirms long-term efficacy and safety profile of iStent implantation in combination with cataract surgery (Neuhann TH et al, Journal of Cataract and Refractive Surgery 2019). The study included 65 eyes of 43 patients with open-angle glaucoma or ocular hypertension. Thirty-eight percent of eyes had undergone prior trabeculectomy and/or glaucoma laser procedures and 68% were on at least two preoperative glaucoma medications.At 5 years postoperative, mean IOP decreased 38% to 14.7 mmHg and topical ocular hypotensive medication use declined 75% while safety profile remained favourable.Additional study findings for eyes followed through five years (n=26 eyes) included:
- A total of 92% of eyes had IOP ≤18 mmHg and 65% had IOP ≤15 mmHg.
- Mean medication use declined 75% to 0.5 topical ocular hypotensive medications, vs. 2.0 preoperatively.
- Approximately 69% of eyes were medication-free vs. 5% preoperatively.
- The safety profile was favorable throughout follow-up.
With over 107 peer reviewed papers on iStent and iStent inject the body of evidence continues to grow and I look forward to sharing more of these with you in the future.
Thank you for your continued support.
Kind regards,
GLENN FAWCETT
General Manager
EXPAND YOUR VIEW
iStent inject is a solution for patients with glaucoma designed to optimise outflow, significantly reduce IOP, provide procedural predictability and precision and prioritising safety. 1,2
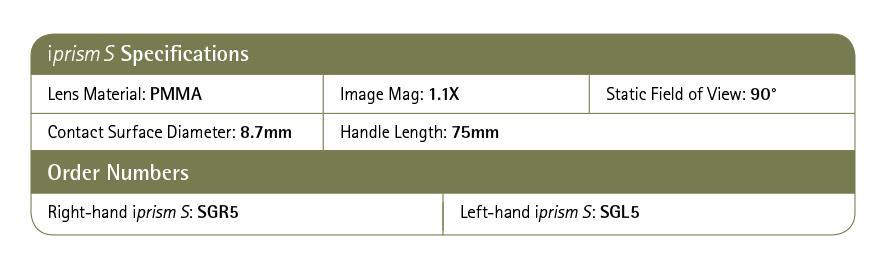
iPrism®S was specifically designed with iStent inject in mind, providing exceptionally wide, crystal clear view through every step of the procedure.
WIDE ANGLE LENS

The broadest field of view to optimise stent placement across multiple clock hours
UNIQUE CONCAVE LENS GEOMETERY

Crystal-clear clarity through a unique lens design, providing an exceptional view every step of the procedure
LIGHTWEIGHT, LOW PROFILE DESIGN
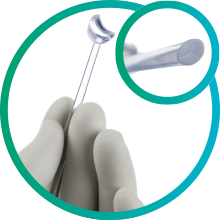
Lightweight, ergonomic design reduces unnecessary pressure on the cornea, minimising striae and incision interference
The iPrism®S is a single-use device that is conveniently packaged and ready for use, eliminating the need for sterilisation and cleaning
Contact your local representative for more information.
REFERENCES: 1. iStent inject ® Trabecular Micro-Bypass System: Directions for Use, Part # 45-0176. 2. Hengerer FH. Personal experience with second-generation trabecular micro-bypass stents in combination with cataract surgery in patients with glaucoma: 3-year follow-up. ASCRS 2018 Presentation.

